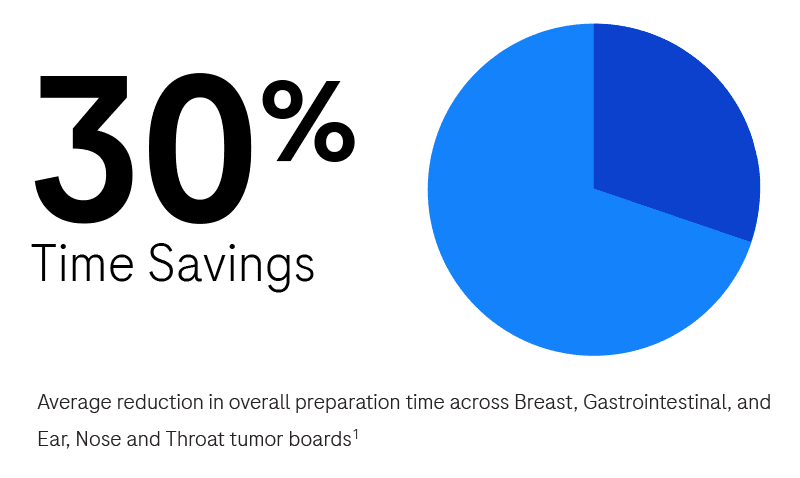
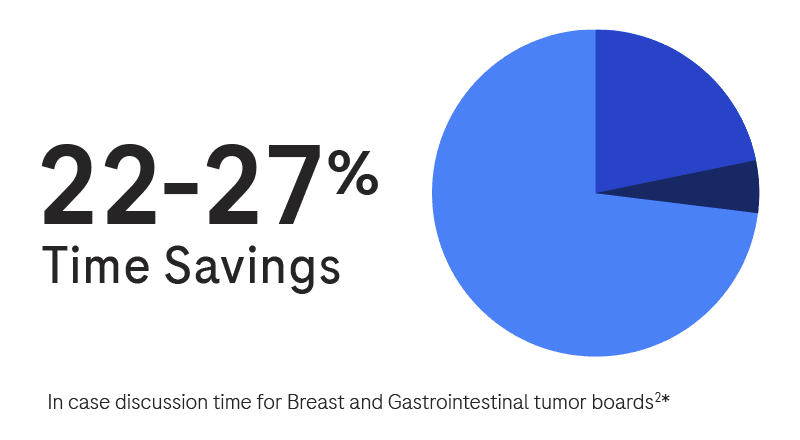
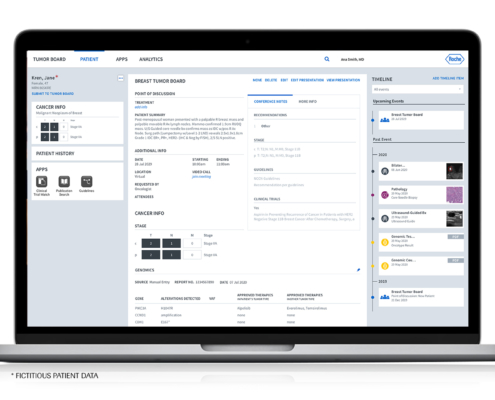
Interested in learning more?
Find us
Roche Information Solutions
Roche Molecular Systems, Inc.
2881 Scott Blvd
Santa Clara, CA 95050
© 2023 Roche Molecular Systems, Inc.
NAVIFY is a trademark of Roche.
All other product names and trademarks are the property of their respective owners.
Not all products of this product portfolio are intended or approved for diagnostic use. This website contains information on products which is targeted to a wide range of audiences and could contain product details or information otherwise not accessible or valid in your country. Please be aware that we do not take any responsibility for accessing such information which may not comply with any valid legal process, regulation, registration or usage in the country of your origin.