Medical University of Vienna, Austria
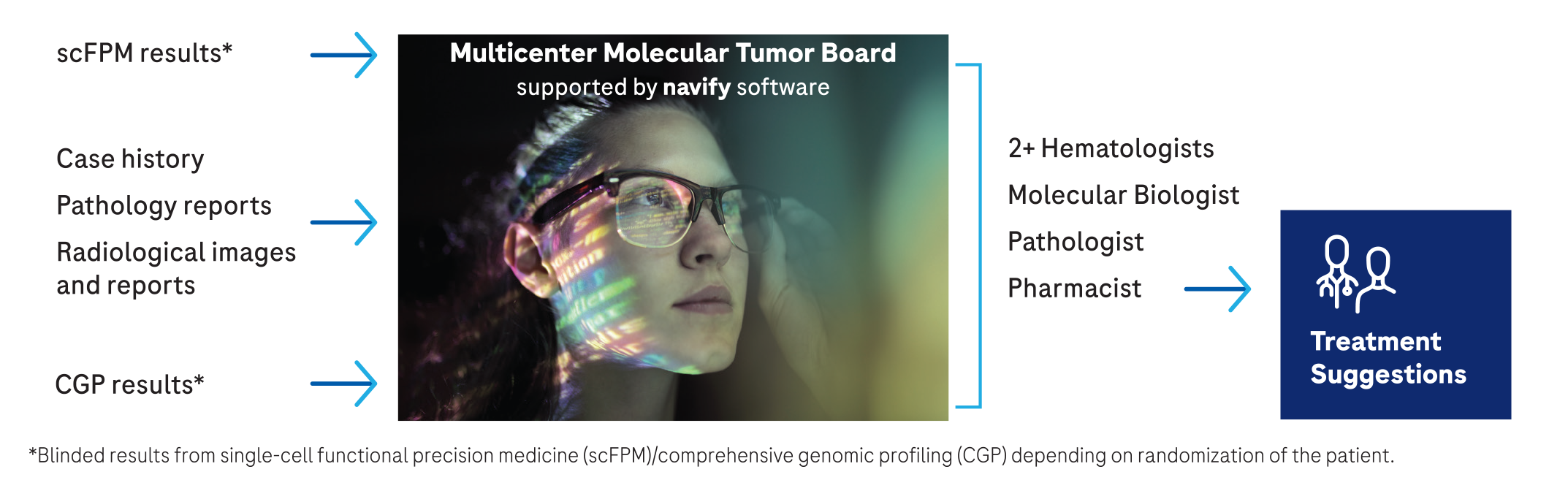
® Tumor Board empowers multi-centric collaboration and consistently robust evaluations to aid clinical researchers in streamlining treatment recommendations
As part of his research laboratory’s focus at Medical University of Vienna, Professor Philipp Staber, MD, PhD, leads his clinical research team in conducting clinical trials to assess novel therapeutic strategies and functional precision medicine approaches in patients with lymphoma, T-PLL, CLL and other hematologic malignancies. In implementing Tumor Board to foster multi-centric collaboration, Professor Staber created a consistent protocol to prepare, conduct and document the tumor board discussions regarding patient participation in clinical trials.